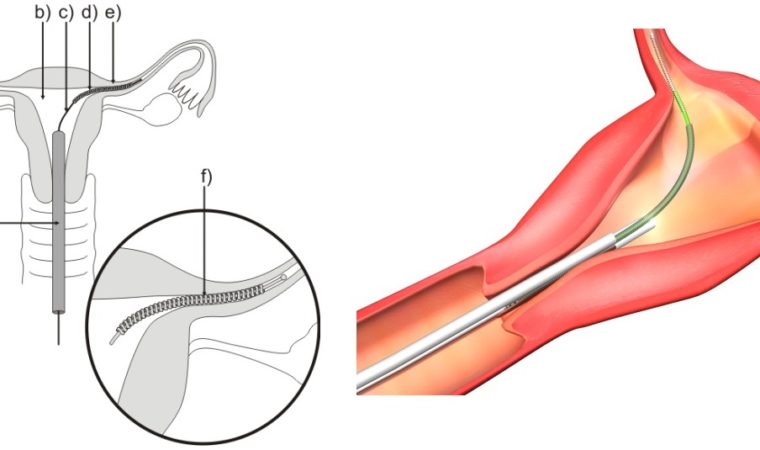
Since Bayer’s sterilization device – also known as a permanent form of birth control – Essure showed up on the medical scene in 2002, it has been surrounded in controversy. Bayer has marketed the device as a low-risk, nonsurgical solution for women who want to avoid pregnancy. The Food and Drug Administration (FDA) is viewing the device in a different light.
Thousands of women have complained that after implantation of the Essure coils, they began experiencing severe symptoms that could be side effects of the device.
Complications reported include:
- Chronic abdominal pain
- Vaginal bleeding
- Severe headaches
- Intense allergic reactions
- Ectopic pregnancy
Getting to the Bottom of the Issue
With so many women reporting complications, the FDA recently conducted a massive survey of more than 50,000 women in total. Participants included 8,000 women whohad the Essure implant, and more than 44,000 women who underwent laparoscopic surgery to block their fallopian tubes. The results from the extensive analysis were alarming.
Although pharmaceutical giant Bayer boasted that women would not need to go into the operating room if they used the Essure device, the study concluded that patients who used Essure were 10 times more likely to require at least one follow-up surgical procedure than women who initiaully underwent laparoscopic surgery.. This translates to more risks and complications, with little to no actual benefit of using the Essure device.
Will Essure Remain on the Market?
Bayer insists that their Essure permanent birth control device has been subjected to decades of testing and is still safe for most women to use. They do concede, however, that their tests did not include results on how the device affected women over 45. In an unfortunate twist, the aforementioned study discovered that women over 40 were the most likely age group to use the device.
The FDA has not seen enough evidence to completely remove Essure from the market. At this point, it seems that the only change coming Bayer’s way may be to add additional warnings surrounding the product. For example, it might not be able to be marketed as a “low-risk” procedure and women would need to be tested ahead of time for certain metal allergies before being allowed to use Essure.
(A full article on the ongoing issue was published by NBC News and may be read here.)
While the FDA continues to look into the troubles caused by Essure, you may need to pursue financial compensation for the pain and suffering it has caused you right away. To work with a legal team that is well-versed with handling cases involving dangerous and defective medical devices, call 704-461-1621 today and speak with a Raleigh personal injury attorney from Whitley Law Firm.


