Smith & Nephew Birmingham Hip Resurfacing System Injury Claims
If you or someone you know has had a metal-on-metal hip implant made by either Smith & Nephew or Biomet fail, we have some good news. It’s now easier to hold these manufacturers of faulty devices accountable—and more victims are joining the cause for defective device justice and compensation every day.
Smith & Nephew Birmingham Hip Resurfacing (BHR) System
Implanted as early as 2009, London-based Smith & Nephew’s BHR R3 acetabular metal liners, part of a ball and socket system, have been used in hip resurfacing and replacement surgery in the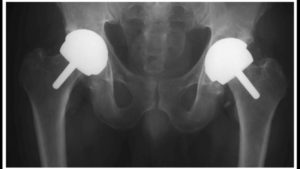 U.S and more than 90 countries worldwide.
U.S and more than 90 countries worldwide.
Some 4,000 people have this brand of metal-on-metal hip system, and unfortunately, there have been a high number of reports of pain and injury from the failing device, along with the revision surgery required to fix the defective implant.
Smith & Nephew recalled the Birmingham Hip Resurfacing metal liner five years after its initial release, when the device began to erode, causing metal particles to gravitate into patients’ blood stream and surrounding tissues. Similar to the dangers with the DePuy ASR device, BHR injuries include tissue and bone death, loosening of the hip joint and dangerous metallosis from metal particles moving through the body. It’s thought that a quarter of all women with BHR hips will experience premature failure due to metallosis.
The good news is all pending federal BHR and R3 defective device cases have now been legally combined in a single venue. This multidistrict litigation not only consolidates and accelerates cases, but also demonstrates to other Smith & Nephew implant victims that lawyers from Whitley Law Firm will continue to hold the manufacturer responsible for improper methodology when creating and selling the medical device.
Our Smith & Nephew Birmingham Resurfacing attorneys are actively looking into any cases related to the Smith & Nephew hip implant. Now is the time to act. Due to the variable state statute of limitations, product liability claims filed too late may not qualify for compensation.
Biomet Sees First Round of Settlements
Biomet metal-on-metal hip implants are also seeing lots of compensation activity, as the first major round of cases on the defective devices have been settled. In fact, since 2011 more than 3,000 people with metal-on-metal Biomet have filed lawsuits against the negligent manufacturer, who is under fired for its unsafe design and marketing of the implant.
Claims, included one formed in Baltimore, MD in April of 2017, are moving forward to recover funds for patients’ medical bills related to medical monitoring; blood tests; revision surgery; physical therapy; and long-term follow-up care.
As we’ve discussed, manufacturer Biomet’s M2a Magnum, M2a 38 and M2a Taper have all shown a higher than average failure rate and have become the subject of lawsuits following the first implant in 2004. Dangerous side effects include metallosis, tissue and bone damage and painful popping, among other issues.
The most important thing? It’s not too late for faulty Biomet hip patients who did not participate in the initial 2004 settlement program between the manufacturer and lawyers representing affected patients. Following this new precedent, Whitely Law Firm would like to talk to any plaintiff with a Biomet-related injury. In fact, hundreds of additional claims are awaiting settlement as we speak.
If you are wondering if you have a case against Biomet, call the defective medical device attorneys at Whitley Law Firm today for more information.


